![]() Nov 26, 2025
Nov 26, 2025
How Electrolyzers Work – The Electrolysis Breakdown
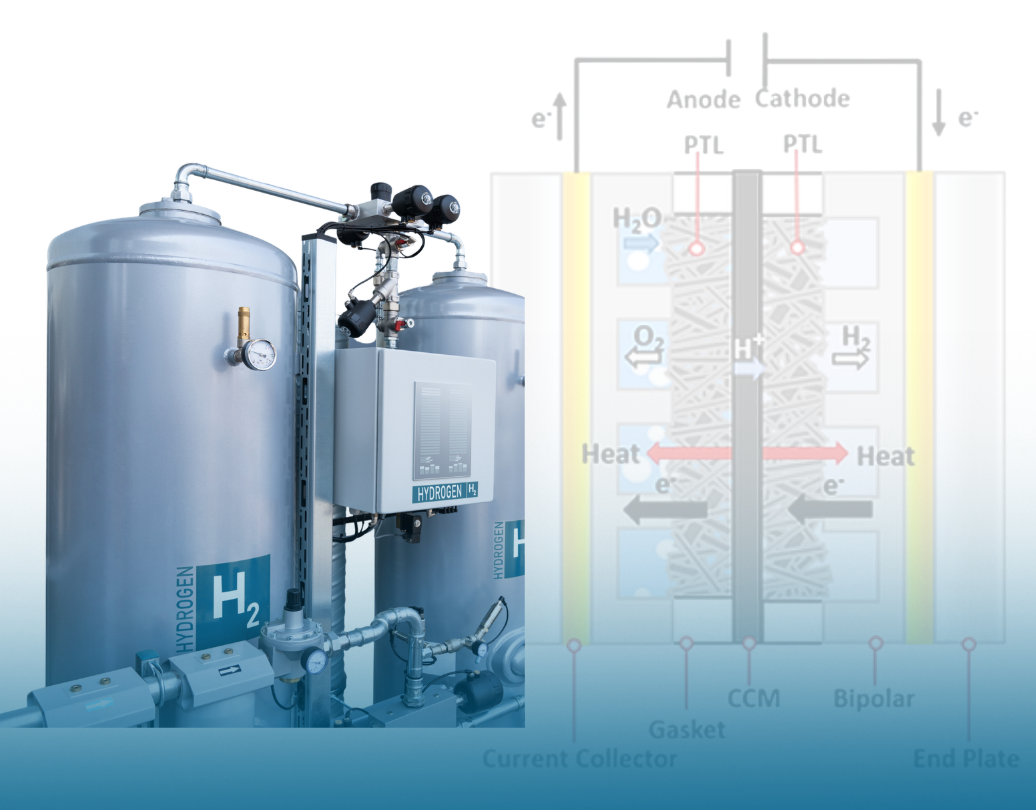
Electrolyzers are at the forefront of technology as industries push for green hydrogen and decarbonization, but how do they actually work? Inside an electrolyzer, a process called electrolysis occurs, which is when electricity is converted into chemical and thermal energy in a single energy cycle through physico-chemical processes.
These processes are the key to creating highly efficient, durable, and environmentally friendly plants. Let’s break them down and explore how they work together to produce green hydrogen.
The Physico-chemical Processes in Electrolyzers
Electrochemistry
Electrochemistry is the first process in the energy cycle and the metaphorical heart of an electrolyzer. It all starts at the cathode and anode, where electrochemical oxidation and reduction reactions take place on the electrode surfaces. These reactions occur in the presence of an electrolyte and under an applied electric potential.
However, since water alone is a poor conductor of electricity, electrolytes like alkalis, acids, or salts are added to enhance its conductivity. In some electrolyzers, special ion-exchange membranes are used to transfer ions between the anode and cathode.

Fig 1. Schematic representation of a membrane
The speed and efficiency of these reactions are vital. The faster hydrogen and oxygen are produced, the higher the electrolyzer’s overall performance. Catalysts and electrode materials also impact performance by reducing energy losses, preventing overheating, and lowering the electrical load. However, when reactions slow down, due to factors like membrane contamination, overall efficiency drops, and the potential for overheating and gas plugging increases.
Overall electrolysis efficiency depends on multiple factors, including temperature and pressure in the cell, the composition and concentration of the electrolyte, electrode material and catalyst, the type of membrane used, and the current density and applied voltage. Now, let’s explore the rest of the processes in the energy cycle.
Diffusion
Diffusion is the transportation of ions and gases and a key process to creating a long-lasting, stable, and efficient electrolyzer. When ions move through the electrolyte, they create a closed electric current. If this movement slows down, then resistance increases and overall performance decreases.
Similarly, gas bubbles must quickly detach from the electrode surfaces. If they linger, they can form a temporary “coating” over the electrodes, blocking the active area and slowing the reactions. Poor bubble removal can also cause uneven diffusion, which contributes to non-uniform membrane and electrode degradation.
Essentially, when this process runs smoothly and the transportation of ions is steady, the electrolyzer can work efficiently for as long as possible.
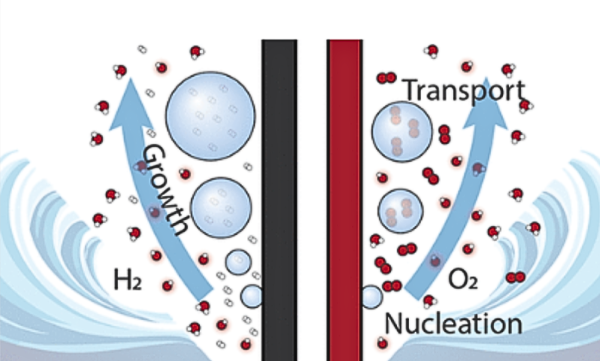
Fig 2. Diagram of gas bubbles generated by the hydrogen evolution reaction and oxygen evolution reaction during water electrolysis
Electroosmosis
Electroosmosis is a behind the scenes process that supports the rest of the energy cycle and keeps the system operating seamlessly. It ensures an even distribution of water and ions, helping reactions remain stable.
This process also enhances the durability and efficiency of an electrolyzer, enabling it to perform consistently at its best.
Heat Transfer
The process of electrolysis generates heat. If this heat is not properly managed, then the temperature of the membrane and catalysts becomes too hot, causing efficiency to drop and increasing the degradation of materials.
That’s why having an effective cooling process is crucial. Heat transfer ensures an optimal temperature for reactions to occur and helps prevent long-term damage. Additionally, the excess heat produced during this process doesn’t go to waste. It can actually be used for energy recovery and boost overall performance and efficiency.
Heat transfer in an electrolyzer is a very important process in the overall energy cycle and plays its unique role in ensuring top system performance.

Fig. 3 Diagram of the Mass / Charge / Thermal conduction
Bringing It All Together: The Complete Energy Cycle
Each process in an electrolyzer is all a part of a coordinated system and they all depend on each other to perform efficiently. Check out Figure 4 for a simple overview of each process as well as their interrelationships and impact on the energy cycle in Figure 5:
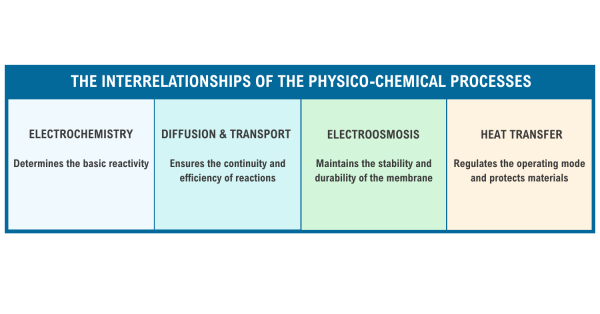
Fig. 4. The Interrelationships of the Physico-chemical Processes
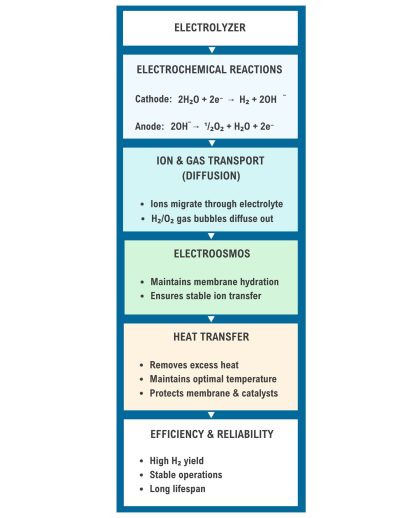
Fig. 5. The physico-chemical processes impact on the energy cycle
Electrolyzer’s Impact on a Cleaner Energy Future
If one of these processes is disrupted, the overall system efficiency drops, hydrogen yield decreases, and the service life of the equipment is reduced. This is why it is so important for each of these processes to run smoothly and cohesively as they all play key roles in ensuring a long-lasting and stable electrolyzer.
Understanding how these physico-chemical processes work together is just the first step in building a cleaner future. The next step is understanding how to integrate them into complex energy systems. In the final blog of this series, we’ll discuss how to practically integrate electrolyzers and how to leverage AxSTREAM System Simulation to model how they interact with an entire system. Stay tuned.
References:
- https://doi.org/10.1016/j.ijhydene.2021.10.166
- https://doi.org/10.1016/j.ijhydene.2021.09.018
- https://doi.org/10.1016/j.energy.2023.128911
- https://doi.org/10.1016/j.rser.2025.116170
- https://doi.org/10.1016/j.rser.2025.116005
- https://doi.org/10.1016/j.rser.2025.116170
- https://doi.org/10.1016/j.jnlest.2021.100080
- https://doi.org/10.1016/j.ijhydene.2019.07.028
- https://doi.org/10.3390/en13184726
- PROSIMPLUS APPLICATION EXAMPLE. HYDROGEN PRODUCTION BY ELECTROLYSIS. Hydrogen production by electrolysis. – March 2024.
- A. Noring, K. Buchheit, and A.K.S. Iyengar, “Techno-Economic Analysis of Large-Scale Hydrogen Production from Solid Oxide Electrolysis Cell Systems,” National Energy Technology Laboratory, Pittsburgh, May 31, 2024.
"*" indicates required fields