![]() Nov 18, 2025
Nov 18, 2025
An Introduction to Modern Electrolyzers and Their Classifications
What if the power of the wind and sun could be used to produce clean fuel from water? This is the idea behind electrolysis, a process that uses electricity to split water (H₂O) into hydrogen (H₂) and oxygen (O₂), effectively converting wind and solar energy into a storable fuel. Modern electrolyzers are driving the production of green hydrogen, particularly when powered by renewable resources.
Hydrogen is emerging as a versatile and sustainable fuel, but realizing its potential requires practical solutions that are efficient, cost-effective, and environmentally friendly. To meet this challenge, researchers are exploring ways to improve electrolysis technologies, tailoring them for different applications and energy sources.
Ongoing efforts use computational and analytical modeling to optimize various types of electrolyzers, including AWE, PEM, SOEC, and AEM, and identify which technologies perform best under specific operating conditions. These efforts are advancing performance and expanding practical applications. Let’s take a deep dive into the growing world of hydrogen energy.
The Push for Hydrogen Energy
Green hydrogen is becoming a cornerstone of the global energy transition. The push is driven by the urgent need to decarbonize industrial processes, transportation, and energy storage. This demand also is intensified by rising fossil fuel prices and stricter low-carbon policies aimed at reducing greenhouse gas emissions [1-4,].
Among the different ways to produce hydrogen, water electrolysis stands out as the most strategic solution. It enables the conversion of surplus renewable electricity into storable hydrogen ‘fuel’. This hydrogen can be used as an energy carrier, injected into natural gas grids, or transformed into valuable chemicals like methanol and ammonia, enhancing its versatility and compatibility with existing energy infrastructures.

Fig. 1. Overview of key hydrogen production pathways and renewable electricity integration.
Figure 1 shows an overview of the key hydrogen production pathways and how they integrate within renewable energy systems.
The History & Evolution of Electrolyzers
Let’s take a walk through the history of electrolyzers. It’s a path of scientific and technological development that started with discoveries in electricity and chemistry in the 18th and 19th centuries, and it’s still unfolding today.
Explore the timeline of electrolyzer development below.

Fig 2. Timeline of electrolyzer development
Types Of Electrolyzers & Their Advantages
Electrolyzers differ widely in design, the type of electrolyte used, and their operating characteristics. Choosing the right electrolyzer depends on specific operating conditions, available resources and energy sources, as well as the required purity and volume of hydrogen produced. With the active development of hydrogen energy and increasing requirements for efficient electrolyzers, greater emphasis is being placed on analyzing and improving existing electrolyzer technologies.
Modern electrolyzers are classified by the type of electrolyte used and the operating temperature range. Each type has its own characteristics, advantages, and limitations. Let’s take a closer look at the most common electrolyzers.
Alkaline Water Electrolyzers (AWE)
Alkaline electrolyzers are the most common type, known for their low cost and easy maintenance. They use a liquid alkaline electrolyte, most often potassium hydroxide (KOH), and are easily scalable for industrial production. These systems are typically used in industries that require a stable, continuous supply of hydrogen for chemical processes, metallurgy, or energy production.

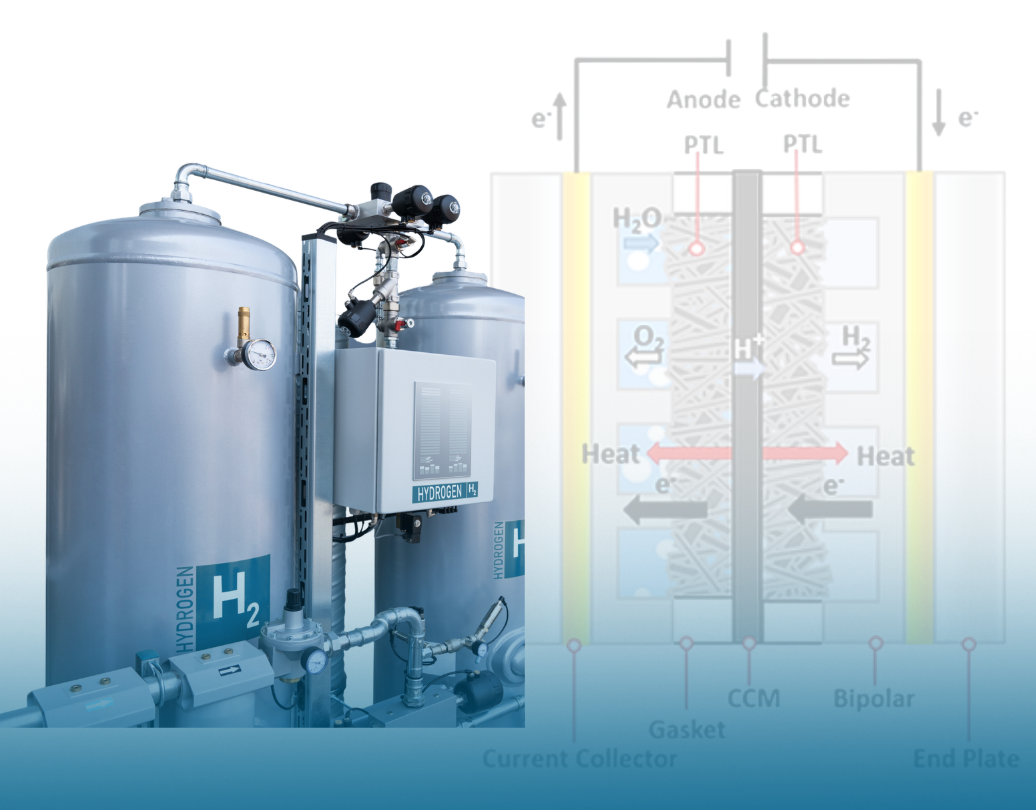
Fig. 3. Electrolyzer cell structure and electrolysis principle [5]
Proton Exchange Membrane (PEM) Electrolyzers
PEM electrolyzers are compact and modular, making them easy to integrate into systems with limited space, such as laboratory research setups and hydrogen-based transportation applications.
They use a polymer exchange membrane instead of a liquid electrolyte, which enhances safety by minimizing the risk of reverse reactions. Because they respond quickly to load changes, PEM electrolyzers are well-suited for unstable energy sources like wind and sun power stations and have a clean output of high-purity hydrogen (>99.9%).

Fig. 4. General principle of operation for PEM electrolysis [6]
Solid Oxide Electrolyzers (SOEC):
SOEC electrolyzers are being actively researched as part of industrial and pilot projects for large-scale production of green hydrogen and combined energy systems. They use a solid ceramic membrane and run at high temperatures (700-900 °C). They can be integrated into industrial processes that generate waste heat, enabling high-pressure hydrogen production without the need for an additional compressor. Thanks to the use of thermal energy, SOEC electrolyzers are highly efficient and reduce electricity consumption.

Fig. 5. Schematic of solid oxide electrolyzer
Anion Exchange Membranes (AEM) Electrolyzers:
AEM electrolyzers are a promising new technology that combines the advantages of alkaline and membrane (PEM) electrolysis, offering high efficiency and made from low-cost materials like nickel and iron. Their compact design and quick response to load changes make them particularly suitable for modular and mobile installations where flexibility and environmental friendliness are important. They use a solid anion exchange membrane where hydroxide ions (OH) are transferred from the cathode to the anode. Since AEM electrolyzers operate in a slightly alkaline environment, corrosion is reduced and overall safety is improved when compared to traditional alkaline systems.

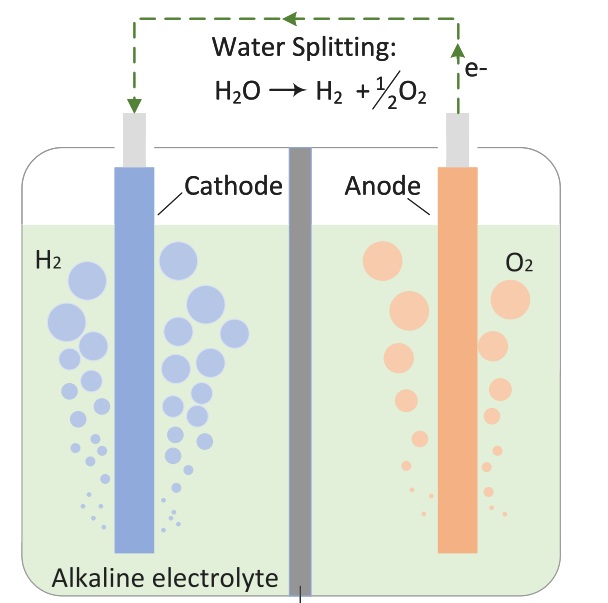
Fig. 6. Schematic of water electrolysis [7]
How Does an Electrolyzer Work?
An electrolyzer works by decomposing water into hydrogen and oxygen through electrochemistry. Inside the device, the electrodes are separated by a special membrane, an electrolyte, that lets ions pass through but not gases.
Reduction occurs at the cathode:
2H2O + 2e− → H2+ 2OH−
This is where pure hydrogen is produced, ready for use in fuel cells and energy generation.
Oxidation occurs at the anode:
2OH− → ½O2 + H2O + 2e−
Oxygen is released, which can either be used or returned to the atmosphere.
But behind this seemingly simple process is a complex system of interrelated physical and chemical phenomena, ranging from precise electrochemical reactions to subtle mechanisms of mass and heat transfer. Take a look at the breakdown of the physico-chemical processes in an electrolyzer.

Fig 7. Physico-chemical processes in electrolyzers
Each of these processes plays an important role in ensuring the efficiency, stability, and durability of the plant.
What’s Next for Electrolysis Technology?
The growing hydrogen market is driving innovation and making clean hydrogen solutions increasingly accessible and scalable. Today, Electrolyzers are not only becoming great technological tools for hydrogen production, but are also key solutions for decarbonization. These trends underscore the importance of a holistic approach, integrating technology, modeling, and market strategies to realize hydrogen’s full potential as a cornerstone of a low-carbon energy future.
Want to dive deeper into the physico-chemical processes in an electrolyzer? Stay tuned for our next blog, where we will explore exactly how electrochemistry, diffusion, electroosmosis, and heat transfer all work together to produce green hydrogen.
References:
- https://doi.org/10.1016/j.ijhydene.2021.10.166
- https://doi.org/10.1016/j.ijhydene.2021.09.018
- https://doi.org/10.1016/j.energy.2023.128911
- https://doi.org/10.1016/j.rser.2025.116170
- https://doi.org/10.1016/j.rser.2025.116005
- https://doi.org/10.1016/j.rser.2025.116170
- https://doi.org/10.1016/j.jnlest.2021.100080
- https://doi.org/10.1016/j.ijhydene.2019.07.028
- PROSIMPLUS APPLICATION EXAMPLE. HYDROGEN PRODUCTION BY ELECTROLYSIS. Hydrogen production by electrolysis. – March 2024.
- Noring, K. Buchheit, and A.K.S. Iyengar, “Techno-Economic Analysis of Large-Scale Hydrogen Production from Solid Oxide Electrolysis Cell Systems,” National Energy Technology Laboratory, Pittsburgh, May 31, 2024.
"*" indicates required fields